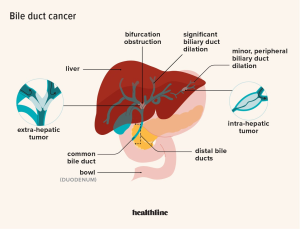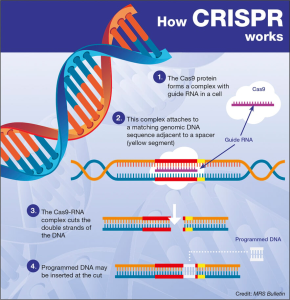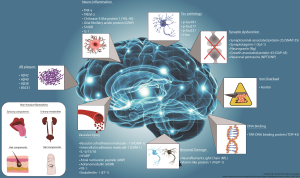
TIM-3 therapy for Alzheimer’s represents a promising new frontier in combating Alzheimer’s disease treatment by re-evaluating the role of the immune system and its impact on neurodegeneration. Recent studies have unveiled that the TIM-3 molecule, an immune checkpoint commonly associated with cancer, can be manipulated to enhance microglia function, thus improving cognitive function in models of Alzheimer’s. Researchers discovered that inhibiting TIM-3 allows microglial cells to effectively clear amyloid plaques—the hallmark of Alzheimer’s—thereby facilitating better memory retention and cognitive performance. This breakthrough not only highlights the intersection between cancer treatment strategies and neurodegenerative disease management but also underscores the importance of the immune system in combating Alzheimer’s. As studies continue to unfold, TIM-3 therapy may soon pave the way for innovative approaches that can significantly alter the course of Alzheimer’s disease in humans.
Exploring novel therapies for Alzheimer’s disease is paramount, and one of the most intriguing developments involves TIM-3, an immune checkpoint molecule. This innovative treatment approach harnesses the body’s immune system to combat the effects of Alzheimer’s, leveraging insights traditionally reserved for cancer treatment. By targeting the TIM-3 pathway, researchers aim to revive the natural functions of microglia, the brain’s immune cells, fostering their ability to eliminate toxic plaque accumulations. This not only pertains to Alzheimer’s disease treatment but also opens a dialogue on how immune system interactions could be pivotal in maintaining memory and cognitive health. Thus, TIM-3 therapy stands out as a potential game changer in both Alzheimer’s and broader neurodegenerative research.
An Overview of Alzheimer’s Disease Treatments
Alzheimer’s disease (AD) is a complex and multifaceted condition that affects millions worldwide. Current treatment options primarily focus on alleviating symptoms rather than curing the disease itself. Traditional therapies have included cholinesterase inhibitors and glutamate regulators, which aim to improve cognitive function and slow down the progression of symptoms. However, these treatments often provide only modest benefits, highlighting the urgent need for alternative strategies that can target the underlying causes of Alzheimer’s.
Recent advancements in understanding the immune system’s role in Alzheimer’s have sparked interest in new therapeutic targets. Research has identified various pathways and mechanisms, such as the role of amyloid-beta plaques and neuroinflammation, that contribute to the disease’s progression. As a result, innovative treatment approaches that leverage immune system modulation are being explored, aiming to not only improve symptoms but also alter the disease course itself.
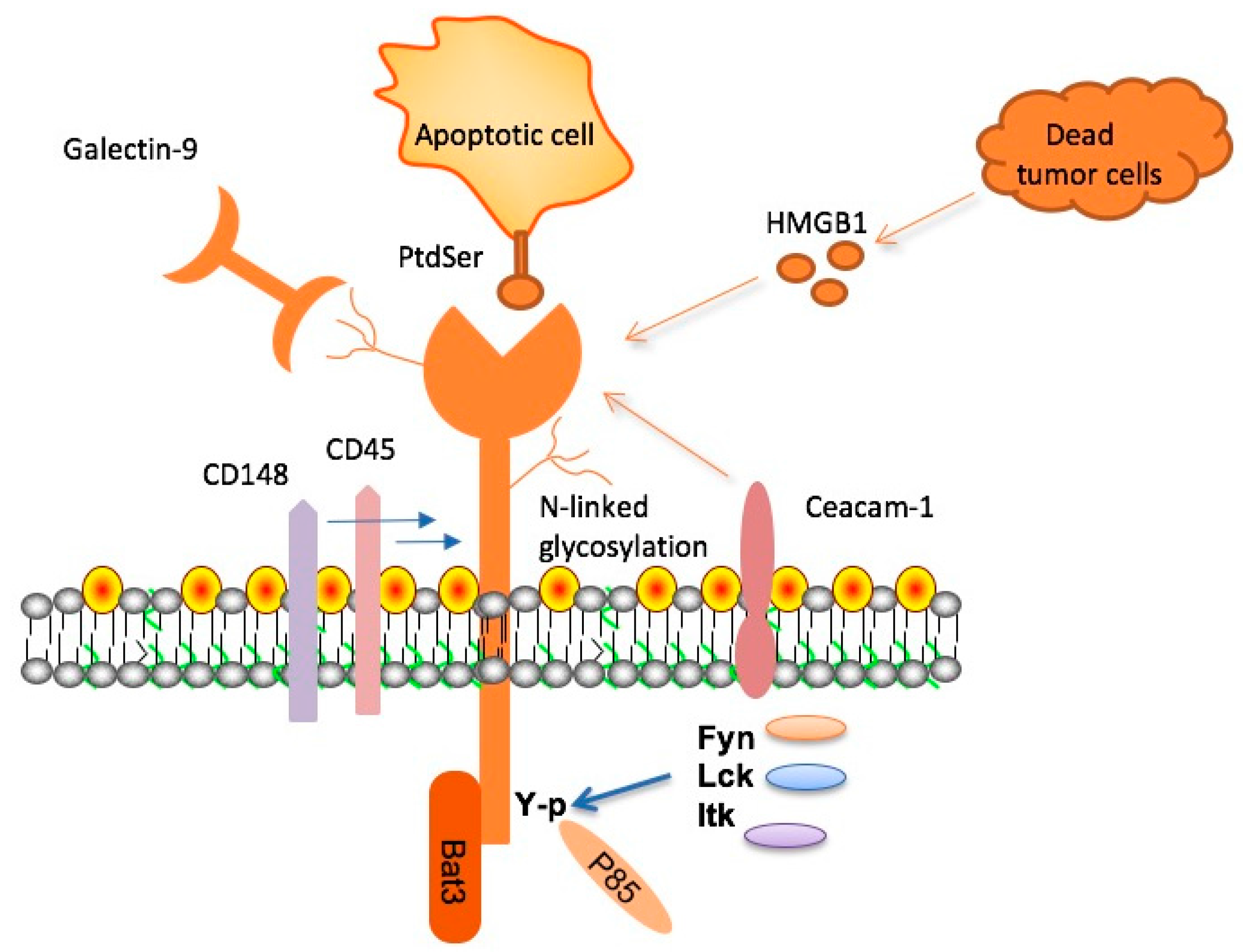
Understanding TIM-3 and Its Role in Alzheimer’s Disease Therapy
Checkpoint molecules, like TIM-3, are vital regulators of the immune system, preventing excessive activation that can cause harm to the body. In the context of Alzheimer’s disease, TIM-3 is primarily expressed by activated microglia, the brain’s resident immune cells. Research shows that high levels of TIM-3 inhibit microglial functions, preventing these cells from effectively clearing amyloid plaques, which contribute to neurodegeneration and cognitive decline.
By exploring TIM-3 therapy for Alzheimer’s, researchers like Vijay Kuchroo emphasize the potential to reverse cognitive impairment by modulating microglial activity. In laboratory settings, deletion of the TIM-3 gene in mice resulted in enhanced plaque clearance and subsequent cognitive improvements. This groundbreaking finding suggests that targeting TIM-3 could lead to innovative therapeutic strategies that not only enhance memory function but also fundamentally change Alzheimer’s disease treatment paradigms.
The Implications of TIM-3 in Microglial Function
Microglial cells serve as the primary immune defense in the central nervous system, playing essential roles in synaptic pruning and the maintenance of brain health. However, in aging and Alzheimer’s disease, these cells can become dysfunctional, leading to an accumulation of neurotoxic plaques. The elevation of TIM-3 expression in microglia serves to inhibit their plaque-clearing abilities, exacerbating the cognitive decline associated with Alzheimer’s.
Research has demonstrated that decreasing TIM-3 expression on microglia permits these cells to more effectively engulf and remove amyloid-beta plaques. This reduction in TIM-3 expression not only restores microglial functionality but also promotes neural repair mechanisms. As such, TIM-3 represents a promising target for therapeutic intervention aimed at enhancing microglial function and ultimately improving cognitive outcomes in Alzheimer’s patients.
Exploring Cancer Treatment Strategies in Alzheimer’s Research
Interestingly, the therapeutic strategies developed for cancer treatment have informed researchers studying Alzheimer’s disease. Checkpoint inhibitors, which have been successful in oncology by unleashing the immune system’s capacity to fight tumors, could similarly enhance microglial responses in Alzheimer’s. By focusing on the TIM-3 signaling pathway, scientists are investigating how these cancer therapies can be repurposed to ameliorate the effects of Alzheimer’s and restore cognitive function.
The cross-disciplinary approach of applying cancer treatment strategies to Alzheimer’s research exemplifies the potential for innovative solutions within complex medical challenges. As more studies reveal the intricate connections between immune response and neurodegenerative diseases, the potential for repurposing existing therapies grows, providing new hope for patients and families impacted by Alzheimer’s.
The Link Between TIM-3 and Memory Preservation
Recent investigations have underscored the critical relationship between TIM-3 expression and memory preservation. In Alzheimer’s disease, the harmful accumulation of amyloid plaques disrupts neuronal communication, leading to memory loss. By modulating TIM-3 expression in microglia, researchers hope to enhance plaque clearance, thus facilitating a return of normal cognitive function. This line of research aligns with broader efforts to develop novel therapies that prioritize memory retention as a key outcome.
Furthermore, the potential to leverage TIM-3 therapy for memory restoration signifies a paradigm shift in Alzheimer’s treatment. Studies showing improved memory-related behaviors in TIM-3 gene-deleted mice illustrate a path forward—highlighting how targeting specific immune regulatory pathways can yield significant cognitive improvements. This indicates that a TIM-3-based therapy could not only halt dementia progression but also reverse some of its cognitive impairments.
The Future of TIM-3 in Alzheimer’s Disease Intervention
As research progresses, the potential for TIM-3 as a therapeutic target in Alzheimer’s disease continues to gain momentum. Developing therapies that can inhibit TIM-3, either with monoclonal antibodies or small molecules, presents an exciting avenue for intervention. The ability to fine-tune the immune response in the brain could lead to breakthrough treatments that not only slow disease progression but actively restore cognitive function.
The future of TIM-3 therapy holds promise, particularly as researchers move towards testing in humanized models. The insights gained from Alzheimer’s mouse models provide a solid foundation for evaluating the efficacy and safety of anti-TIM-3 therapies in humans. As we continue to bridge the gap between basic research and clinical application, TIM-3 could play a crucial role in reshaping Alzheimer’s disease management.
Potential Side Effects and Considerations for TIM-3 Therapy
While TIM-3 offers a promising target for Alzheimer’s treatment, it is essential to consider the potential side effects associated with manipulating immune checkpoint pathways. Overactivation of microglial functions may lead to unintended neuroinflammation or other risks, necessitating a careful balance between enhancing immune responses and preventing excessive activity. Understanding the complexities of TIM-3’s role will be crucial in developing safe and effective therapies.
Additionally, as researchers explore different TIM-3 blocking strategies, identifying the optimal timing and dosage for administration will be critical for minimizing adverse effects. Close monitoring of patient responses will be necessary to ensure that interventions yield the desired cognitive benefits while maintaining overall brain health.
Research Collaboration in TIM-3 Studies
The journey towards developing TIM-3 therapies for Alzheimer’s involves extensive collaboration among scientists, clinicians, and institutions. Significant efforts at places like Harvard Medical School, where experts from various disciplines converge, facilitate a holistic approach to tackling Alzheimer’s disease. Collaborative research fosters innovation by combining diverse expertise, ultimately driving the development of comprehensive treatment protocols.
Cooperation between research groups also enhances the ability to secure funding and resources necessary for long-term studies. The sharing of data and findings accelerates the translation of research discoveries into clinical applications that can positively impact patients suffering from Alzheimer’s disease. As collaborations expand, the potential for breakthroughs in TIM-3 therapy becomes increasingly promising.
Regulatory Pathways for TIM-3 Therapies
As TIM-3 therapies move closer to clinical trials, understanding regulatory pathways will be essential for bringing innovative treatments to market. Navigating the complex landscape of drug approval requires adherence to rigorous safety and efficacy standards. Researchers must work closely with regulatory agencies to ensure that therapies utilizing TIM-3 modulation are thoroughly evaluated for their impact on Alzheimer’s disease and broader neuroimmune interactions.
Addressing regulatory challenges will also involve transparent communication about study results, including both successes and setbacks. Regulatory bodies typically emphasize the relevance of preclinical and early-phase trial data in determining the safety profiles of new treatments. Engaging with regulators early in the development process can streamline pathways to approval and ultimately facilitate timely access for patients in need of effective Alzheimer’s treatments.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s focuses on targeting the TIM-3 molecule, which inhibits the activity of microglia, the brain’s immune cells. By blocking TIM-3, these cells can better clear amyloid-beta plaques linked to Alzheimer’s disease, potentially improving cognitive function and memory.
How has TIM-3 been linked to Alzheimer’s disease treatment outcomes?
Research indicates that TIM-3 is expressed at higher levels in microglia of Alzheimer’s patients, contributing to plaque accumulation. By utilizing TIM-3 therapy, scientists aim to rejuvenate microglia function, enabling them to clear plaques and enhance memory, thus improving Alzheimer’s treatment outcomes.
What role do microglia play in the effectiveness of TIM-3 therapy for Alzheimer’s?
Microglia are crucial for the clearance of amyloid-beta plaques in the brain. TIM-3 therapy aims to enhance their function by inhibiting the TIM-3 molecule, allowing microglia to effectively attack plaques and restore cognitive abilities in Alzheimer’s disease.
What type of research supports the use of TIM-3 therapy for Alzheimer’s disease?
Recent studies, including one published in Nature, demonstrate that deleting the TIM-3 gene in mice led to improved cognition and plaque clearance. This research supports TIM-3 therapy as a promising strategy for Alzheimer’s disease treatment.
Could TIM-3 therapy provide insights into treating Alzheimer’s alongside other methods?
Yes, TIM-3 therapy could offer a complementary approach to existing Alzheimer’s treatment strategies, such as anti-amyloid therapies, by enhancing the immune response in the brain and addressing plaque-related cognitive decline.
What are the potential benefits of using anti-TIM-3 antibodies for Alzheimer’s treatment?
Anti-TIM-3 antibodies may selectively inhibit the negative effects of TIM-3 on microglia, potentially allowing better plaque clearance and improved memory function, which could lead to significant advancements in Alzheimer’s disease treatment.
Are there any risks associated with TIM-3 therapy for Alzheimer’s disease?
While TIM-3 therapy holds promise, potential risks must be assessed, including the impact on immune regulation in the brain and the possibility of overactive immune responses leading to inflammation.
When can we expect TIM-3 therapy for Alzheimer’s disease to be available?
TIM-3 therapy for Alzheimer’s is still in research stages, with ongoing testing in mouse models. It will require further clinical trials to determine its safety and efficacy before it becomes widely available as a treatment option.
How does TIM-3 therapy compare to traditional Alzheimer’s treatments?
Unlike traditional Alzheimer’s treatments that primarily focus on managing symptoms, TIM-3 therapy offers a potential mechanism to directly enhance the brain’s immune response to clear plaques, potentially leading to more substantial improvements in cognitive function.
Where is TIM-3 research currently being conducted for Alzheimer’s therapy?
Research on TIM-3 therapy for Alzheimer’s is being conducted at leading institutions, including Harvard Medical School and Brigham and Women’s Hospital, where scientists are investigating its efficacy in animal models and planning future human trials.
| Key Points | Details |
|---|---|
| Study Subject | TIM-3 therapy for Alzheimer’s disease. |
| Research Findings | Deletion of TIM-3 in mice allowed microglia to clear Alzheimer’s plaques, leading to improved memory. |
| Significance | TIM-3 is a genetic risk factor for late-onset Alzheimer’s and has implications for new treatments. |
| Mechanism | TIM-3 inhibits microglia from clearing amyloid-beta plaques, which accumulate in the brain. |
| Future Implications | Potential for TIM-3 blocking therapies in human patients, possibly improving cognitive function. |
Summary
TIM-3 therapy for Alzheimer’s disease presents a promising avenue for treatment by targeting immune system mechanisms that hinder plaque clearance in the brain. Recent studies highlight the role of TIM-3 as an inhibitory checkpoint molecule that prevents microglia from attacking harmful amyloid-beta plaques. By manipulating this pathway, researchers have observed increased clearance of these plaques in mouse models, resulting in improved memory and cognitive function. This innovation could lead to breakthroughs in therapeutic approaches aimed at mitigating the impacts of Alzheimer’s and restoring cognitive abilities.