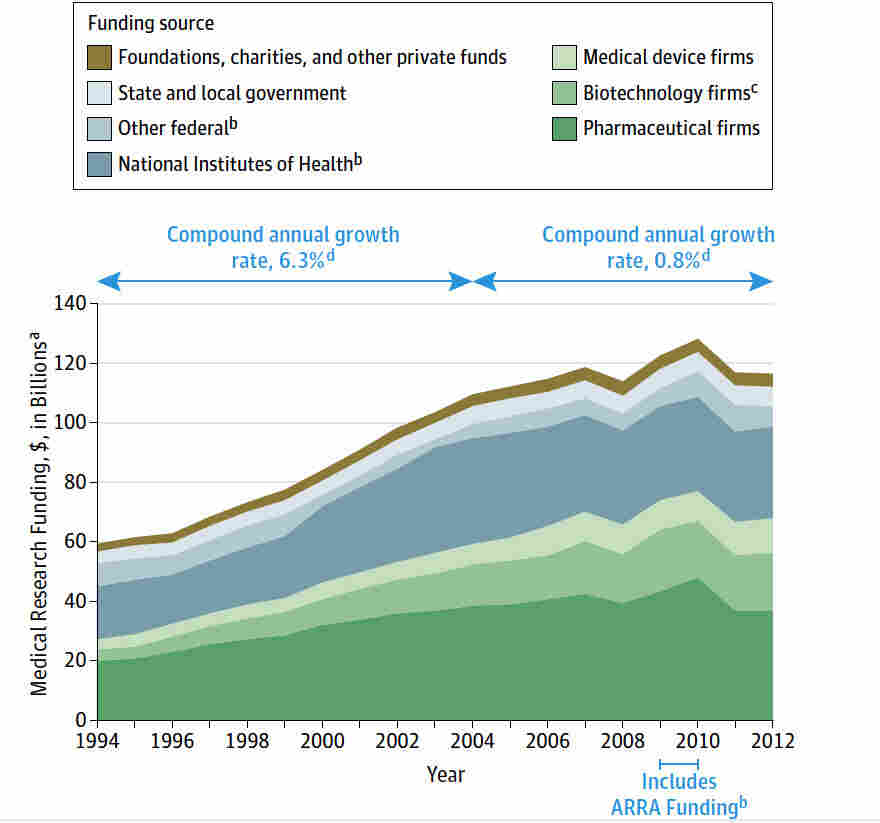
Medical research funding is a critical element in advancing healthcare and ensuring patient safety. Without adequate financial support, research initiatives suffer, which can undermine clinical research oversight and compromise the well-being of participants. The recent cuts in IRB funding have raised alarms across the medical community, signaling potential setbacks in collaborative research efforts designed to protect human subjects. As institutions grapple with the limitations imposed by these funding shortages, the potential impact on NIH grant opportunities could hinder innovation and delay the development of vital treatments. Ultimately, the stability of medical research funding is essential, not only for progressing scientific discovery but also for maintaining trust in the healthcare system.
Securing financial resources for medical studies represents a foundational aspect of health advancement and participant welfare. This funding is pivotal for facilitating thorough reviews and oversight of clinical trials, ensuring that ethical standards are upheld. In light of recent funding decrements affecting institutional review boards, concerns arise regarding collaborative efforts aimed at safeguarding research subjects. The ramifications of reduced NIH investment can significantly stifle the momentum of groundbreaking medical investigations, leaving vital questions about patient protection unanswered. A stable funding landscape is essential for the continued integrity and safety of medical research endeavors.
The Importance of Medical Research Funding for Patient Safety
Medical research funding plays a crucial role in ensuring the safety and well-being of patients involved in clinical trials. Without sufficient financial resources, institutions may struggle to maintain the rigorous oversight required to protect participants. Federal funding, particularly from organizations like the National Institutes of Health (NIH), underpins the infrastructure that allows researchers to adhere to ethical guidelines and regulations. This includes the necessary reviews and continuous monitoring facilitated by Institutional Review Boards (IRBs), which evaluate the safety protocols in place. When funding is cut, the capacity for these essential protective measures is significantly diminished, potentially placing patients at risk.
Moreover, disruptions in funding can lead to halted studies and limited ability to recruit new participants, thereby extending timelines and delaying advancements in medical treatments. For instance, collaborative research projects often require the engagement of multiple sites to gain a comprehensive understanding of a therapeutic’s effectiveness. When grants are cut, researchers face barriers to expanding their studies, resulting in incomplete data and a failure to meet patient safety standards. Consequently, the halt in funding not only restricts immediate research but can also erode public trust in the medical research community, placing vulnerable populations at further risk.
Impact of IRB Funding Cuts on Clinical Research Oversight
Funding cuts to IRBs threaten the backbone of clinical research oversight by jeopardizing the resources necessary for the comprehensive evaluation of study proposals. IRBs are charged with ensuring that research protocols uphold participants’ rights by assessing the research’s ethical implications, safeguarding issues, and informed consent processes. Without adequate funding, IRBs may experience staffing shortages or lack the capability to conduct thorough reviews, which can lead to inadequate protections for participants. This domino effect not only jeopardizes the safety of those involved but also risks compromising the integrity of clinical research as a whole.
Additionally, as collaborative research expands, the demand for efficient IRB processes grows. The shift towards utilizing single IRBs (sIRBs) to oversee multisite studies is intended to streamline approval processes and enhance patient safety. However, without sufficient funding, even these sIRB systems may struggle to function effectively. The delays and operational capacity issues stemming from funding cuts can severely disrupt research timelines, affecting the development and delivery of new treatments to patients who urgently need them. Ultimately, sustaining adequate IRB funding is paramount for preserving both ethical standards in clinical research and the safety of participants involved.
Collaborative Research Issues and Their Repercussions on Patient Safety in Medical Trials
Funding cuts can exacerbate collaborative research issues, complicating the process for conducting multicenter trials that are vital for comprehensive patient safety evaluations. When various institutions pool their resources and expertise, they can address complex health challenges more effectively, enhancing the quality of care provided to patients. However, when federal funding is interrupted, institutions may be reluctant to engage in collaborative studies, fearing the financial burden or reduced oversight capabilities. This hesitation can stall innovative approaches to patient safety, preventing transformative treatments from reaching the market.
Furthermore, these funding disruptions can lead to decreased enrollment in clinical trials. As potential participating institutions withdraw due to uncertainties about the stability of funding or the effectiveness of IRB processes, participants may have fewer opportunities to engage in vital research. This lack of participation not only undermines the diversity of study populations but also stifles the development of therapies that are inclusive and representative of all patient demographics. By detracting from collaborative efforts, funding cuts ultimately disturb the delicate equilibrium necessary for successful clinical research, thereby undermining patient safety outcomes.
The Broader Implications of NIH Grant Impact on Medical Studies
The impact of NIH grant funding extends far beyond individual studies; it fundamentally influences the overall landscape of medical research and patient care. When funding is disrupted, it can create a trickle-down effect that hampers both ongoing and future medical investigations. Research requires a substantial amount of time and resources, and without consistent grant allocations, institutions may be forced to scale back their ambitions. This reduction in research capabilities can lead to slower progress in understanding and treating diseases that directly affect patient health and safety.
Moreover, cuts in NIH funding can stymie innovation as researchers become less able to pursue ground-breaking ideas that require significant investment. As competition for limited resources intensifies, researchers may feel compelled to conform to traditional lines of inquiry rather than taking risks on innovative approaches. This stagnation in research can have adverse consequences for patient safety, as the newest therapeutic developments may be put on hold, delaying vital solutions to pressing health challenges. Ultimately, the ramifications of NIH grant impacts highlight the delicate balance between funding availability and the advancement of medical research, directly correlating funding stability to patient outcomes.
Historical Context: The Evolution of Medical Research Standards
Understanding the historical context of medical research is essential for appreciating the evolution of standards that safeguard patient rights and safety. Events such as the Tuskegee Syphilis Study have significantly shaped public perceptions and regulatory frameworks surrounding clinical research. In response to these ethical breaches, policies and oversight mechanisms were developed to ensure rigorous protections were in place. Today, the relevance of maintaining these standards cannot be overstated, particularly in light of federal funding cuts that threaten the integrity of oversight systems.
As we reflect on the lessons of the past, it is clear that we must remain vigilant in ensuring that such tragedies do not recur. The establishment of IRBs and subsequent regulations was not merely a bureaucratic measure but a crucial safeguard against potential harm to research participants. If funding continues to dwindle, the systems established to protect individuals participating in clinical research are at risk of degradation. Fostering a culture that prioritizes ethical research practices will require sustained investment and commitment from all stakeholders involved in the medical research community.
Navigating Collaborative Research in the Face of Funding Challenges
The landscape of collaborative medical research is being reshaped by funding challenges that impact both researchers and participants. Collaborative research efforts are crucial for tackling complex health issues, enabling institutions to pool expertise and resources. However, when funding is uncertain, collaborative partners may second-guess their involvement, hesitant to commit to projects that could be jeopardized by potential budgetary cuts. This reluctance can stifle innovation and significantly limit the number of viable studies aimed at improving patient outcomes.
The need for stable funding becomes especially evident as we seek to address pressing public health challenges. When researchers collaborate effectively, they harness diverse perspectives that enhance the study design and analytical rigor. Yet, as funding becomes limited, the rigidity of traditional models re-emerges, often deterring researchers from pursuing innovative methodologies. Thus, nurturing an environment that encourages collaboration not only aids in participant recruitment but also strengthens the review processes that ensure patient safety in research studies.
The Role of Patient Engagement in Medical Research Funding
Engaging patients as active participants in research is becoming increasingly recognized as vital for improving study design and outcomes. Yet, financial constraints can inhibit efforts to foster this engagement, which is essential for ensuring that research aligns with patient needs and preferences. Funding limitations can prevent institutions from implementing patient-centered research frameworks, which would allow patients to contribute meaningfully throughout the research process. As a result, key stakeholder perspectives may remain unaddressed, which can lead to gaps in understanding patient experiences and expectations.
Moreover, when patients are involved in research planning and oversight, it enhances the accountability of researchers to prioritize safety and ethics. Their insights can help refine protocols and address potential concerns before studies commence. However, when funding is cut, initiatives aimed at promoting this engagement may falter, thereby reducing the research community’s ability to facilitate informed consent and address issues of safety adequately. It is paramount for agencies to recognize the interdependence between funding, patient engagement, and overall research integrity to bolster trust and improve patient outcomes in clinical studies.
Addressing the Future of Medical Research Amid Funding Cuts
As the landscape of medical research evolves amidst funding cuts, stakeholders must actively advocate for the sustainability of essential resources. Ensuring patient safety in clinical trials necessitates a robust funding environment that supports the ethical oversight mechanisms, including IRBs, that have become integral to research integrity. As financial constraints threaten to disrupt these systems, the medical community must come together to address these challenges and emphasize the critical importance of research funding.
Strategies for securing ongoing funding must include strengthening advocacy efforts for federal grant allocations and creating partnerships with private sectors to diversify funding streams. Additionally, addressing the public’s perception of medical research can help garner support for funding initiatives, highlighting how investments in research translate into safer and more effective health solutions for individuals and communities. Truly, the future of medical research depends on committing to principles that prioritize patient safety and innovation through sustainable financial support.
Frequently Asked Questions
How does medical research funding impact patient safety in clinical trials?
Medical research funding plays a crucial role in ensuring patient safety during clinical trials. Federal funds, especially from the National Institutes of Health (NIH), support the operational costs associated with Institutional Review Boards (IRBs), which oversee research proposals. These IRBs ensure that studies comply with regulations aimed at protecting participants’ rights and welfare. Without adequate funding, oversight could be compromised, potentially leading to increased risks for patients involved in medical research.
What are the consequences of IRB funding cuts on patient safety in medical research?
IRB funding cuts can severely impact patient safety in medical research by limiting the resources available for thorough review and oversight of studies. Such cuts can lead to reduced staffing or operational capabilities, making it difficult for IRBs to assess risks associated with clinical trials adequately. This insufficiency may compromise the ethical standards of research and ultimately endanger the well-being of participants.
How do NIH grants influence collaborative research issues in medical studies?
NIH grants are vital for addressing collaborative research issues by providing necessary funding for multi-site studies. These grants facilitate the implementation of single IRB review processes, which streamline oversight for collaborative projects across various institutions. By securing NIH funding, researchers can more effectively work together while ensuring that patient safety protocols are upheld, helping to maintain public trust in medical research.
What role does medical research funding play in ensuring clinical research oversight?
Medical research funding ensures robust clinical research oversight by empowering IRBs to perform their essential functions. Funding allows IRBs to hire qualified professionals who monitor research for compliance with ethical standards and participant safety regulations. As a result, well-funded oversight mechanisms help reassure the public about the integrity and safety of clinical trials.
What impact do funding cuts have on the safeguards implemented for patient safety in medical research?
Funding cuts directly threaten the safeguards established for patient safety in medical research. With diminished resources, IRBs and research institutions may struggle to conduct thorough reviews of studies, leading to potential gaps in monitoring research protocols. This risk can diminish the efficacy of protections designed to prevent harm and ensure ethical treatment of participants.
How might reduced medical research funding affect patient outcomes in clinical trials?
Reduced medical research funding can negatively affect patient outcomes in clinical trials by leading to inadequate oversight and monitoring of research activities. When IRB functions are compromised, there is a greater risk of ethical violations and harm to participants, which can decrease the overall reliability of clinical trial results. This decline in oversight can lead to suboptimal patient care and erosion of trust in the research process.
In what ways do collaborative research issues arise from medical research funding challenges?
Collaborative research issues often arise from medical research funding challenges when institutions are unable to secure the necessary resources for coordinated studies. Funding cuts can hinder the ability of multiple sites to participate in a study effectively, delaying research progress and complicating logistics. These challenges not only impact the research timeline but also the safety protocols that are vital for protecting patient welfare.
How does medical research funding help prevent the ethical pitfalls associated with historical medical experiments?
Medical research funding helps prevent ethical pitfalls associated with historical medical experiments by supporting the establishment and functioning of IRBs, which enforce strict ethical guidelines. Adequate funding enables ongoing education and training for researchers about ethical practices, ensuring lessons learned from past abuses are upheld in modern research environments, thereby protecting current and future study participants.
| Key Point | Details |
|---|---|
| Funding Cuts | The Trump administration’s halt of $2 billion in federal research grants to Harvard disrupts patient safety and rights in medical research. |
| SMART IRB System | SMART IRB enables oversight of research across multiple sites and is essential for compliance and patient safety. |
| Role of IRBs | Institutional Review Boards (IRBs) provide ethical oversight, ensuring the safety and rights of research participants. |
| Historical Context | Past medical abuses have necessitated strong oversight measures, leading to the establishment of IRBs in the U.S. |
| Impact of Halts | Halting studies risks harming participants and erodes public trust in research. |
Summary
Medical research funding is crucial for safeguarding patients in clinical studies. The recent freeze on funding has significant implications not only for the continuation of essential research but also for the protections afforded to individuals who participate in these studies. The absence of funds disrupts oversight and protections in research processes, posing risks to participant safety and well-being. This highlights the pressing need for sustained medical research funding to uphold ethical standards and ensure public confidence in medical advancements.