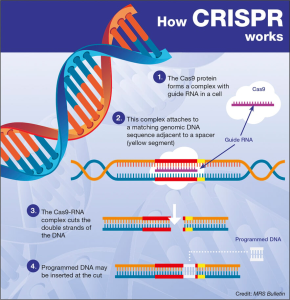
CRISPR gene editing has revolutionized the way we think about genetic disorders and disease treatment, offering unprecedented potential to alter the very fabric of life. With its ability to edit genes at the most fundamental level, this technology holds promise for conditions like sickle cell disease, which have long plagued families and stifled health equity. However, the ethical implications of gene therapy, such as germline editing concerns and the broader societal impacts, have sparked intense debate among experts. As discussions continue about the ethical issues of gene editing, particularly those surrounding the rights of parents to choose genetic traits for their children, it is essential to approach advancements in this field with caution. The intersection of scientific innovation and moral responsibility underscores the need for comprehensive oversight as we navigate the future of CRISPR.
The emergence of CRISPR technology, often referred to as genetic scissors, is fundamentally changing how we understand and treat hereditary diseases. This gene-editing tool enables precise modifications at the genetic level, paving the way for revolutionary advances in medicine, particularly in treating conditions such as sickle cell anemia. Yet, alongside its capabilities, there arise significant conversations about the ethics of gene manipulation and the responsibility that comes with such power. The discourse around genetic modifications often delves into issues including life quality, societal implications, and health equity, prompting critical examination of who gets access to these transformative procedures. As we explore the future of germline and somatic cell editing, it is vital to ensure that ethical considerations are at the forefront of innovation.
The Promise of CRISPR Gene Editing
CRISPR gene editing represents a revolutionary advancement in the field of genetics, offering unprecedented opportunities to cure diseases previously deemed incurable. With its ability to precisely alter genetic sequences, CRISPR has generated hope for effectively treating conditions like sickle cell disease, a devastating ailment affecting thousands. By enabling targeted gene modifications, CRISPR allows for the removal of faulty genes responsible for such diseases, thereby potentially eradicating the symptoms and improving the quality of life for countless patients.
However, as we stand on the brink of scientific breakthroughs, it is crucial to consider the ethical ramifications of utilizing such technology. The ability to edit genes raises profound questions about the limits of human intervention in nature. While curing diseases is a noble pursuit, it compels us to evaluate the broader implications of altering human genetics and the slippery slope it may create regarding designer babies or gene enhancements.
Ethical Dilemmas of Gene Editing
The deployment of CRISPR technology is fraught with ethical dilemmas that scholars and medical professionals are wrestling with. One significant concern revolves around the idea of germline editing, which not only affects the individual being treated but can also have ramifications for future generations. This ability to create genetic changes that are heritable raises critical questions about the nature of human evolution and what it means to be human. Should we allow parents to decide the genetic traits of their offspring, especially in cases where conditions are not life-threatening? The decision-making power in these scenarios is ambiguous and laden with moral complexities.
Additionally, there are pressing health equity issues that arise from the use of CRISPR gene editing. The high costs associated with gene therapies, such as the $2.2 million treatment for sickle cell disease, present significant barriers to access. This raises significant concerns regarding who can benefit from these advancements and whether they will exacerbate existing health disparities. As stated by ethicists and health professionals, the implications of such technologies must be navigated carefully to ensure that innovation does not simply enhance the lives of the privileged few.
Sickle Cell Disease Treatment: A Case Study
Sickle cell disease is a prime example illustrating the potential benefits and ethical quandaries of CRISPR gene editing. With CRISPR’s capacity to remove the genes that cause sickle cell disease, we stand on the threshold of curing thousands of affected individuals. For patients who have endured the debilitating effects of this chronic condition, such advancements offer a light at the end of the tunnel. As researchers explore these gene-editing techniques, the prospect of a normal, healthy life free from the restraints of sickle cell disease is an undeniable allure.
However, the application of this technology also prompts critical discussions about the ethical responsibility of scientists and healthcare providers. The emotional weight of curing such a profoundly challenging condition must be weighed against the moral duty to consider the implications of gene editing not just on the individual, but on society at large. Will we risk moving down a path leading to genetic inequality, where only those who can afford treatment can access life’s possibilities?
Germline Editing Concerns and Their Implications
Germline editing presents one of the most contentious aspects of CRISPR technology, sparking debates about its implications for future generations. When editing genes that are passed down to offspring, we must consider not just the immediate outcomes but the legacy we create. This technology opens up a Pandora’s box of possibilities where genetic traits can be selected for or against, leading to ethical concerns about eugenics and the right to an unaltered genetic identity. The risks of unforeseen consequences in the editing of germline cells call for rigorous discussions about regulatory oversight and moral responsibility in the scientific community.
Moreover, the debates surrounding germline editing emphasize the importance of creating equitable standards for evaluating these modifications. Conversations about who decides which traits are desirable or undesirable are critical, especially when considering the diverse spectrum of human conditions. The potential for societal pressure to conform to genetic idealism may lead to an erosion of the appreciation for human diversity, complicating our understanding of health equity and genetics.
Gene Therapy Ethics: A Balancing Act
As gene therapy becomes more mainstream, the ethical considerations surrounding its implementation grow more complex. While the potential to eradicate genetic disorders suggests a positive impact, ethical frameworks must be established to govern its application. Questions regarding informed consent, the potential commodification of genes, and the implications for future generations must all be thoroughly examined. The ability to manipulate human DNA urges us to create systems that prioritize ethical considerations alongside scientific ambitions.
Additionally, the notion of modifying human genes for therapeutic purposes intersects with broader societal themes, including access to care and socioeconomic disparities. As more individuals pursue gene therapies, ensuring fair access for all, regardless of economic status, remains a pressing challenge. The ethics of gene therapy thus extends beyond individual treatment to encompass broader societal implications, urging healthcare systems to seek solutions that uphold health equity amid rapid advancements in technology.
Health Equity in the Era of Gene Editing
The intersection of health equity and gene editing highlights the crucial need for inclusive dialogue as biotechnology evolves. High costs associated with treatments like CRISPR technologies can create a divide between those who can afford such transformative therapies and those who cannot. This disparity raises questions about who ultimately benefits from innovations in healthcare, as financial barriers could preclude vulnerable populations from receiving advancements that could significantly enhance their quality of life.
As discussions unfold regarding CRISPR and similar technologies, it is imperative that policymakers, healthcare stakeholders, and ethicists prioritize strategies that promote equitable access to modern medical advancements. Addressing health equity concerns will require a concerted effort to establish policies that ensure widespread availability of gene editing solutions, thereby preventing the exacerbation of existing health inequalities and promoting justice in the realm of genetic healthcare.
The Future of Gene Editing and Society
Looking ahead, the future of gene editing will undoubtably be shaped by ongoing societal discussions around ethical practices and implications of CRISPR technology. As the potential for gene editing to address genetic disorders becomes more pronounced, society must grapple with the responsibilities that come with such power. Engaging in conversations about the ethical boundaries of genetic manipulation will be essential in navigating potential pitfalls and unforeseen consequences that may arise.
The necessity for interdisciplinary collaboration among scientists, ethicists, policymakers, and communities is paramount to addressing the complexity of gene editing technology. The dialogue surrounding the application of CRISPR and gene therapies should not only focus on the health benefits but also involve comprehensive discussions about the ethical, social, and cultural implications of altering human genetics to foster an inclusive future that respects human diversity and autonomy.
The Role of Oversight in Gene Editing
Effective oversight is crucial in the regulation of gene editing practices, particularly as technologies like CRISPR advance rapidly. The ability to manipulate human genes raises pressing questions about how to safeguard against unethical practices, ensure safety, and prevent misuse of such powerful technologies. Balancing innovation with oversight will be essential for fostering public trust in gene editing advancements while mitigating risks associated with unregulated practices.
International collaboration will also play a pivotal role in establishing robust regulatory frameworks as gene editing transcends national borders. Ethical adherence to universal standards can help curb potential abuses, such as conducting germline editing in countries with lax regulations. Leaders, scientists, and ethicists must join forces to create comprehensive guidelines that encompass ethical principles and safety protocols for utilizing gene editing technologies responsibly.
Community Perspectives on Gene Editing
Incorporating community perspectives into discussions about gene editing offers a broader understanding of how technology impacts different populations. Engaging with diverse communities will shed light on varying ethical viewpoints and experiences related to genetic conditions. This inclusive dialogue can lead to more equitable outcomes in gene therapy by acknowledging the unique concerns and cultural beliefs present within different communities.
Moreover, elevating community voices in conversations about CRISPR technologies could foster increased public awareness and comprehension of genetic innovations. By informing community members about the possibilities and ethical considerations surrounding gene editing, we can create informed advocates for equitable healthcare that drives responsible advancements in gene therapies and treatment methods.
Frequently Asked Questions
What are the ethical issues of gene editing with CRISPR?
CRISPR gene editing raises several ethical issues, such as the potential for germline editing, which can alter the DNA of future generations. Concerns include the moral implications of modifying human traits and the risk of unforeseen consequences on human genetics. Additionally, there are debates surrounding health equity, as access to these advanced treatments may be limited for underprivileged populations.
How does CRISPR gene editing potentially treat sickle cell disease?
CRISPR gene editing enables the manipulation of genes responsible for sickle cell disease by either repairing or disabling the faulty gene in somatic cells. This approach can significantly improve patient outcomes and potentially cure the disease. However, the ethical question remains if such interventions should be pursued given the high costs and accessibility issues surrounding CRISPR treatments.
What are the concerns regarding germline editing in CRISPR technology?
Germline editing using CRISPR technology involves altering the genetic information in sperm, eggs, or embryos, leading to permanent changes in subsequent generations. Concerns center around the potential for ‘designer babies,’ inequalities in access to gene editing, and the risks associated with unintended genetic consequences. Ethical discussions also revolve around who decides which traits are desirable.
How is health equity impacted by CRISPR gene editing technologies?
Health equity is significantly impacted by CRISPR gene editing technologies due to the high cost of treatments and the potential for unequal access based on socioeconomic status. As advanced gene therapies like those for sickle cell disease emerge, it raises concerns about who will afford these life-changing interventions and whether such disparities will create a wider gap in health outcomes.
What ethical questions arise from the CRISPR gene editing of traits that enhance human capabilities?
The use of CRISPR for gene editing traits that enhance human capabilities presents ethical questions about parental rights and societal norms. For instance, if parents wish to modify traits such as intelligence or physical ability, it raises issues of consent for the child. Furthermore, this could lead to societal divisions based on genetic enhancements, prompting debates on the morality and consequences of such practices.
How do unintended consequences of CRISPR technology pose ethical challenges?
Unintended consequences of CRISPR technology pose ethical challenges as gene alterations may lead to unforeseen health issues or genetic disorders that were not anticipated. This complexity necessitates thorough oversight and research to ensure the safety and efficacy of gene editing interventions, which adds layers of ethical considerations regarding human experimentation and the responsibility of scientists and institutions.
| Key Points | Details |
|---|---|
| Ethical Dilemmas | Discussion revolves around the morality of changing human differences, especially in cases like sickle cell anemia. |
| Somatic vs Germline Gene Editing | Somatic gene editing affects existing cells; germline editing affects embryo genes, with potentially permanent effects. |
| Costs and Accessibility | The sickle cell cure costs around $2.2 million, raising questions about who can afford it and health equity. |
| Variations in Ethics Across Diseases | Concerns about editing for conditions like Down syndrome and the authority of parents in such decisions. |
| Oversight and Regulation | Lack of international monitoring on gene editing practices, especially in countries with loose regulations. |
| Unintended Consequences | Gene edits may have unforeseen effects due to the complex nature of genetic interactions. |
Summary
CRISPR gene editing represents a revolutionary technology in medicine, offering potential cures for genetic diseases like sickle cell anemia. However, this innovation also brings forth significant ethical questions regarding the implications of altering human genetics. The discussions led by experts emphasize the need to balance medical advancements with ethical considerations, particularly concerning access to treatments, the risk of unintended genetic consequences, and the moral responsibilities of parents in deciding the traits of future generations.