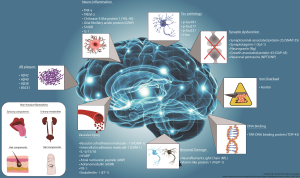
Alzheimer’s disease research is at the forefront of neuroscience, particularly through the groundbreaking work of Beth Stevens, an esteemed investigator who explores the critical role of microglial cells in the brain’s immune system. These cells are responsible for maintaining brain health by removing debris and refining synapses, the vital connections that relay information between neurons. However, Stevens’ research reveals that improper synapse pruning can contribute to the progression of Alzheimer’s and other neurodegenerative diseases. By delving into this fundamental aspect of brain function, her team aims to uncover new biomarkers and potential therapies that could transform the lives of millions affected by these conditions. With support from the NIH and a commitment to curiosity-driven science, Stevens is paving the way for a deeper understanding of how our brains protect themselves and, ultimately, how we can protect them.
Research into Alzheimer’s disease encompasses the exploration of various neurological conditions, highlighting the intricate mechanisms behind cognitive decline. Terms like neurodegenerative disorders and brain immune responses are central to understanding the dynamics at play within the affected populations. Investigators such as Beth Stevens focus on the role of specialized cells, called microglia, which play a pivotal part in the health of brain synapses through a process known as synaptic pruning. This fundamental research not only sheds light on the vast complexities of brain functions but also opens avenues for developing new treatment strategies aimed at combating memory disorders. By addressing the underlying biological factors, we can hope to make significant progress in managing and ultimately eradicating the impacts of Alzheimer’s and related conditions.
Understanding Microglial Cells in Alzheimer’s Disease Research
Microglial cells have emerged as crucial players in the landscape of Alzheimer’s disease research. These specialized immune cells monitor the brain’s environment, responding to injury and disease by clearing cellular debris and modulating neuronal connections through a process known as synapse pruning. When functioning correctly, microglia protect brain health and contribute to maintaining cognitive functions. However, recent studies suggest that improper microglial activity may actually accelerate neurodegeneration, emphasizing the need for deeper insights into their role in Alzheimer’s and other neurodegenerative diseases.
Beth Stevens’ groundbreaking work focuses on understanding these microglial cells and their implications in Alzheimer’s disease. Her findings have brought to light the intricate balance between synapse formation and pruning, which microglia naturally regulate. When this balance is disrupted, it can lead to impaired cognitive functions. Through rigorous research supported by NIH grants, Stevens and her team are paving the way for therapeutic approaches that target microglial dysfunction, marking significant progress in the quest to treat Alzheimer’s disease more effectively.
The Role of Synapse Pruning in Neurodegenerative Diseases
Synapse pruning is a fundamental process in brain development and functionality, involving the elimination of excess synapses to refine neural circuits. This process is vital for learning and memory, but its dysregulation has been linked to various neurodegenerative diseases, including Alzheimer’s, Huntington’s disease, and schizophrenia. Recent research reveals that mismanaged synapse pruning by microglial cells can degrade neural pathways, contributing to cognitive decline. Therefore, understanding the mechanisms driving synaptic health is critical for developing effective interventions for these devastating conditions.
Beth Stevens’ cutting-edge research delves into the complexities of synapse pruning and its implications for neurodegenerative diseases. By employing innovative experimental models, her lab has identified pathways that may lead to potential biomarkers for early detection. This focus on synaptic health not only advances our understanding of Alzheimer’s disease but also opens doors to new therapeutic strategies aimed at modulating microglial activity to restore synaptic balance and improve cognitive function.
Frequently Asked Questions
How do microglial cells influence Alzheimer’s disease research?
Microglial cells are pivotal in Alzheimer’s disease research as they comprise the brain’s immune system. They monitor neuronal health and remove damaged cells, playing a crucial role in synapse pruning. In studies led by Beth Stevens, improper microglial function has been linked to neurodegenerative diseases like Alzheimer’s, highlighting their importance in developing new therapies.
What is synapse pruning and its relevance to neurodegenerative diseases?
Synapse pruning is a process where microglial cells refine and eliminate weak synapses to enhance neural connectivity. This mechanism is critical in Alzheimer’s disease research, as improper synapse pruning can lead to cognitive decline. Research by Beth Stevens has shown that disruption in this process may contribute to various neurodegenerative diseases, paving the way for potential treatments.
Who is Beth Stevens and what is her contribution to Alzheimer’s disease research?
Beth Stevens is a prominent neuroscientist whose research has transformed our understanding of microglial cells in Alzheimer’s disease. Her work at Boston Children’s Hospital focuses on how these immune cells affect synaptic connectivity and their role in neurodegenerative diseases. Stevens’ insights are vital for developing new biomarkers and therapies for Alzheimer’s.
How does the research on microglial cells relate to the treatment of Alzheimer’s disease?
Research on microglial cells is critical for developing treatments for Alzheimer’s disease. By understanding their role in synapse pruning and response to neural injury, scientists like Beth Stevens can identify potential therapeutic targets. This research aims to correct the dysregulation of microglial functions, offering hope for effective Alzheimer’s therapies.
What insights have been gained from studying the brain’s immune system in relation to neurodegenerative diseases?
Studying the brain’s immune system, particularly microglial cells, has provided significant insights into neurodegenerative diseases such as Alzheimer’s. Research led by Beth Stevens indicates that these cells play a dual role: protecting the brain while also contributing to disease when their function is altered. This knowledge is crucial for developing targeted therapies.
Why is foundational research important in Alzheimer’s disease studies?
Foundational research is essential in Alzheimer’s disease studies as it lays the groundwork for understanding complex biological processes. As Beth Stevens emphasizes, initial curiosity-driven research enables scientists to explore mechanisms, like microglial interactions and synapse pruning, that have direct implications for treating neurodegenerative diseases.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, monitoring health and repairing damage. |
| Synapse Pruning | Improper pruning may lead to Alzheimer’s and other neurological disorders. |
| Funding and Support | Research backed by NIH has been pivotal in advancing Alzheimer’s disease research. |
| Foundational Research | Basic science is essential for discovering treatments that can improve lives. |
Summary
Alzheimer’s disease research is crucial for understanding and combating this devastating illness. The work of scientists like Beth Stevens highlights the pivotal role that microglial cells play in brain health and disease. As research continues to uncover the mechanisms by which improper synapse pruning can lead to neurodegenerative diseases, it becomes clear that thorough and persistent investigation into the brain’s immune responses is necessary. With the support of organizations like the NIH, breakthroughs in understanding these processes could pave the way for new treatments, ultimately offering hope to millions affected by Alzheimer’s.